The Regulation of Ph by Cells Is Accomplished Primarily Through
The regulation of pyruvate kinase involves phosphorylation by a kinase pyruvate kinase kinase resulting in a less-active enzyme. Phosphate is useful in animal cells as a buffering agent and the most common form is HPO 2 4.

Acid Base Balance Anatomy And Physiology Ii
It gets more complicated with larger organisms where cells are not in direct contact with the outside environment.
. In order for vitamin D to become active it must undergo a hydroxylation reaction in the kidney that is an OH group must be added to calcidiol to make calcitriol 125-dihydroxycholecalciferol. Level constancy is accomplished primarily through negative feedback systems which ensure that blood glucose concentration is maintained within the normal range of 70 to 110 milligrams 00024 to 00038 ounces of glucose per deciliter approximately one. This effect is accomplished through modulating the activity of motor proteins by the salmonella pathogenicity island 2 SPI-2-encoded type III secretion system effector protein SifA which forms.
Most of the control of the respiration processes is accomplished through the control of specific enzymes in the pathways. Pyruvate kinase is also regulated by ATP a negative allosteric effect. Understanding physiology can only be accomplished through the study of evolution by natural selection.
Lungs alter pH through changes in exhaled CO2. Physiologic pH where pH stands for potential of hydrogen is a way of quantifying the balance between acids and bases in the body. Overall concepts will be emphasized rather than.
In these highly differentiated cells pHiregulation is accomplished through sarcolemmal acidbase transport proteins and intracellular buffers working in concert with the diffusive movement of protons coupling the two processes Vaughan-Jones et al 2006a. Long-Term Regulation of Low BP Long-term regulation of blood pressure is primarily accomplished by altering blood volume. Changes in intracellular pH are a cells response to externally applied agents such as hormones growth factors and others.
Of pH regulation in the secretory pathway. The affect a chemical messenger elicits in a cell is determined primarily by the identity of A. In cardiac myocytes the trans-sarcolemmal extrusion of acid equivalents in response to an.
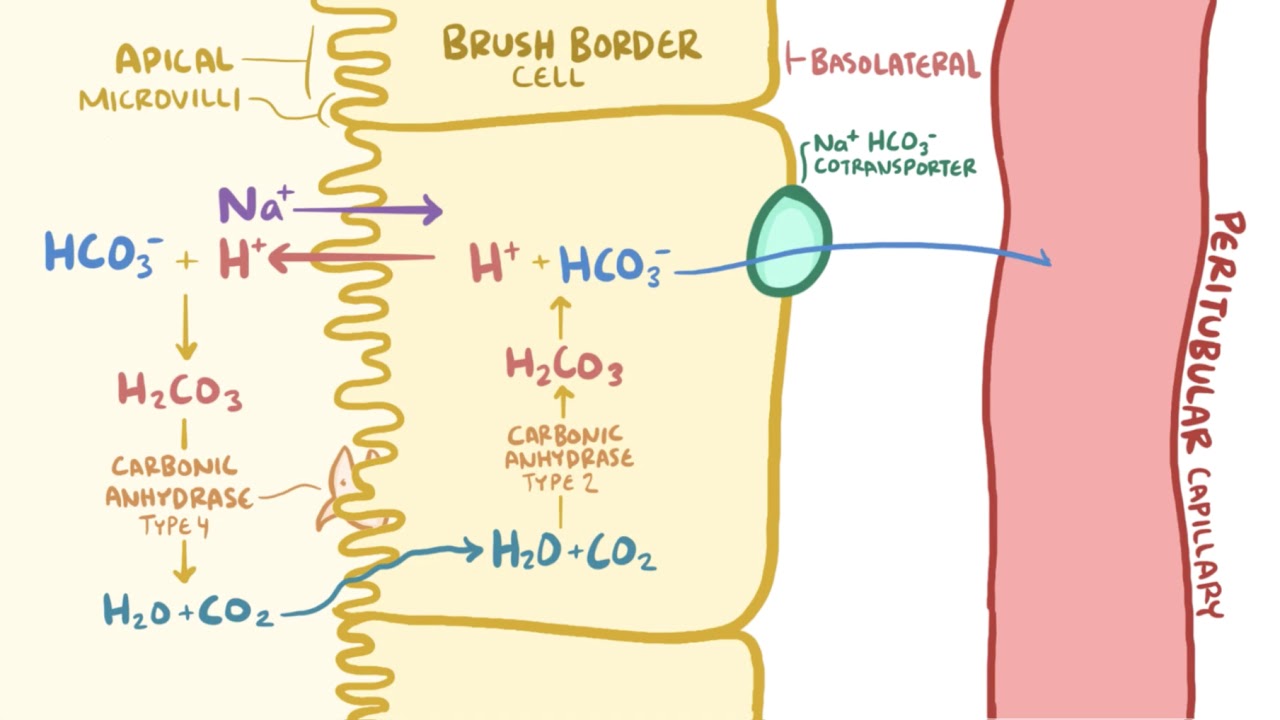
The renal system can also adjust blood pH through the excretion of hydrogen ions H and the conservation of bicarbonate but this process takes hours to days to have an effect. PH regulation by neurons and glial cells involves a variety of membrane acidbase carriers including sodiumhydrogen exchange sodiumbicarbonate cotransport and sodium-dependent and sodium-independent chloridebicarbonate exchange. Cellular respiration is controlled by a variety of means.
On a cellular level many essential cellular processes metabolic enzymes and transmembrane transport processes are highly pH sensitive. Measuring pH in the Secretory Pathway The simplest and most convenient method to measure organellar pH is through the targeted delivery of pH-sensitive spectroscopic probes. The regulation of pHi is achieved through the actions of Na H ion exchangers and other pumps.
In fact the pH depends on the concentration of hydrogen ions and can be described with this equation. Buffers are chemical substances that bind with hydrogen and hydroxyl ions and keep them from causing big changes in pH. Overall concepts will be emphasized rather than.
This is accomplished via processes that exchange molecules across the cell membrane. Although this review will address systemic pH regulation and the role of the kidneys individual cells also have a variety of mechanisms to regulate their intracellular pH. For a single-celled organism this is accomplished through direct exchange with the environment.
Animal cells can function only within a narrow nearly neutral range of internal pH pHi. Transport of acidbase equivalents across the cell membranes of neurons and glial cells also results in pH changes in the. This is a type of negative feedback.
Indeed this approach has proven fruitful in The pH of the Secretory Pathway. Cell volume regulation. Dephosphorylation by a phosphatase reactivates it.
An early response thereof in most tissues is a rapid cytoplasmic acidification of roughly half a pH unit. The regulation of pH by cells is accomplished primarily through buffers. The buffer systems functioning in blood plasma include plasma proteins phosphate and bicarbonate and carbonic acid buffers.
On a cellular level many essential cellular processes metabolic enzymes and transmembrane transport processes are highly pH sensitive. Cells under anoxia experience an energy crisis. Bicarbonate profile is similar to Na.
Bicarbonate major 60 carrier of CO2 in blood. The entry of glucose into a cell is controlled by the transport proteins that aid glucose passage through the cell membrane. The loss of blood through hemorrhage accident or donating a pint of blood will lower blood pressure and trigger processes to restore blood volume and therefore blood pressure back to normal.
This is accomplished primarily through alterations in sodium and water reabsorption the mechanisms of which differ within each nephron segment. The kidneys in concert with neural and endocrine input regulate the volume and osmolality of the extracellular fluid by altering the amount of sodium and water excreted. The cytoplasm has buffer systems that help to keep intracellular pH within the neutral rangeThis is important because the end products of many metabolic reactions in cells.
The cells and enzymes in our tissues and organs work best when the concentration of hydrogen ions is 40 x 10-9 nEqL. As pH falls the 3 factors involved in increased H excretion are. Measurement Determinants and Regulation.
Kidneys alter pH by adjusting levels of bicarbonate. Background pH regulation is the result of a complex interaction of ion transport H buffering H -consuming and H -producing reactions. The addition and removal of phosphate from the proteins in all cells is a pivotal strategy in the regulation of metabolic processes.
Although this review will address systemic pH regulation and the role of the kidneys individual cells also have a variety of mechanisms to regulate their intracellular pH. Increased ammonium excretion increases steadily with decrease in urine pH and this effect is augmented in acidosis This is the major and regulatory factor because it can be increased significantly. Regulation of cellular pH B.
Proper kidney function is essential for pH homeostasis. Bone and teeth bind up 85 percent of the bodys phosphate as part of calcium phosphate salts.

The Role Of The Kidney In Acid Base Balance Osmosis

Gut Microbiota And Its Possible Relationship With Obesity Mayo Clinic Proceedings In 2022 Gut Microbiota Intestinal Microbiota Obesity

No comments for "The Regulation of Ph by Cells Is Accomplished Primarily Through"
Post a Comment